|
Centaurea solstitialis
|
|
|
|
Scientific name
|
Centaurea solstitialis
|
|
Additional name information:
|
L.
|
|
Common name
|
yellow starthistle
|
|
Synonymous scientific names
|
none known
|
|
Closely related California natives
|
0
|
|
Closely related California non-natives:
|
11
|
|
Listed
|
CalEPPC List A-1,CDFA C
|
|
By:
|
John D. Gerlach Jr.,Joseph M. DiTomaso
|
|
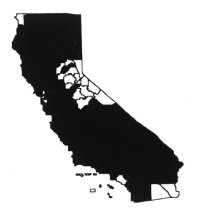
Distribution
|
|
HOW DO I RECOGNIZE IT?
Distinctive features:
|
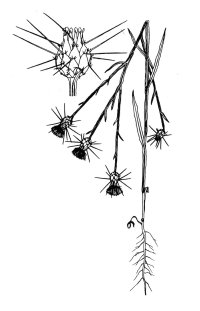
In California, yellow starthistle
(Centaurea solstitialis) grows as a deep-taprooted winter annual, or
rarely as a short-lived perennial. It produces one to many solitary, spiny,
yellow flower-heads during late spring, summer, and fall. Seeds begin to
germinate soon after fall rains, and young plants grow as prostrate to ascending
taprooted rosettes until bolting occurs in late spring or early summer. Stem
leaves of bolted plants extend downward, giving the stems a winged appearance.
Flowering plants range from ankle to shoulder height and change color from green
to bluish green in summer. Flowerheads are generally produced from June through
September. The heads are initially produced on branch tips, but robust plants
may produce heads in the branch axils later in the season. The main phyllaries
(flowerhead bracts) are palmately spined with a single stout, apical spine and a
few much smaller, lateral spines. Some individuals produce shorter apical
spines. The heads contain two types of fruits or achenes. Most are cream to tan
with a white pappus or plume; achenes in the outer ring are darker and lack a
pappus.
|
|
Description:
|
Asteraceae. Annual. Stems:
6-72 in (15-200 cm) in height. Leaves: basal, earliest, entire to slightly
toothed; subsequent, lobed to deeply lobed; bright green and
scabrous-bristly in seedling and rosette stages; 2-6 in (5-15 cm) long;
cauline leaves long, entire, narrow, decurrent; initially green, becoming
bluish green and densely covered with cobwebby hairs later in the season;
leaf blades 0.4-1.2 in (1-3 cm) long. Inflorescence: produced late
May-December; heads 1 to many, always solitary; involucre 0.5-0.7 in
(13-17 mm) tall, ovoid; outer phyllaries with apical appendages palmately
spiny, central spine 0.4-1 in (10-25 mm) long, generally stout; tips of
inner phyllaries with membranous winged tips about 1 mm wide. Flowers:
many; corollas 0.5-0.8 in (13-20 mm) tall, unusually equal, yellow;
marginal florets sterile, corollas 2-4 lobed, spreading to ascending;
inner florets fertile, 5 lobed. Fruits: achenes 0.08-0.12 in (2-3 mm)
long; those produced by outer ring of flowers dull, dark brown to
blackish, without pappus; those produced by interior flowers glossy,
grayish to mottled light brown; pappus white with bristles 0.08-0.166 in
(2-4 mm) long, pappus bristles covered with rows of minute barbs; achene
attachment scar obtuse, achene base broad (Hickman 1993, Gerlach unpubl.
data). |
|
|
|
WHERE WOULD I FIND IT?
|
Yellow starthistle is most widely
distributed in the Sacramento and northern San Joaquin valleys, Inner North
Coast Ranges, northern Sierra Nevada foothills, Cascade and Klamath ranges, and
the central-western regions of the state (Hickman 1993). There are many small to
large relict populations in the southwestern region of California. It is
currently spreading in mountain regions of the state below 7,500 feet (2,250 m)
and in the central-western region. It is uncommon in deserts and at moist
coastal sites. Primarily it is a problem in moderately warm, exposed areas on
fertile, drier soils, including disturbed sites, grasslands, rangeland, hay
fields, pastures, roadsides, and recreational areas (DiTomaso et al.
1999).
|
|
WHERE DID IT COME FROM AND HOW IS IT SPREAD?
|
Yellow starthistle is native to southern
Europe and western Eurasia and was first collected in Oakland, California, in
1869. It was most likely introduced after 1848 as a contaminant of alfalfa seed.
Introductions prior to 1899 were most likely from Chile, while introductions
from 1899 to 1927 appear to be from Turkestan, Argentina, Italy, France, and
Spain (Gerlach in prep., Hillman and Henry 1928). By 1917 it had become a
serious weed in the Sacramento Valley and was spreading rapidly along roads,
trails, streams, ditches, overflow lands, and railroad rights-of-way (Newman
1917). In 1919 Willis Jepson observed its distribution near Vacaville and
stated: ÛÏIt is 1,000 times as common as ten years ago, perhaps even six years
agoÛ (Jepson 1919).
Yellow starthistle had spread
to over a million acres of California by the late 1950s and nearly two million
acres by 1965. In 1985 it was estimated to cover eight million acres in
California (Maddox and Mayfield 1985) and perhaps ten to twelve million acres a
decade later. It is equally problematic around Medford in southwestern Oregon
and in HellÛªs Canyon in Oregon and Idaho (Maddox et al. 1985). It also infests,
to a lesser degree, areas in eastern Oregon, eastern Washington, and Idaho
(Roch̩ and Roch̩ 1988).
Human activities are the
primary mechanisms for the long-distance movement of yellow starthistle seed.
Seed is transported in large amounts by road maintenance equipment and on the
undercarriage of vehicles. The movement of contaminated hay and uncertified seed
is also an important long-distance transportation mechanism. Once at a new
location, seed is transported in lesser amounts and over short to medium
distances by animals and humans. The short, stiff, pappus bristles are covered
with microscopic, stiff, appressed, hair-like barbs that readily adhere to
clothing and to hair and fur (Gerlach unpubl. data). The pappus is not an
effective long-distance wind-dispersal mechanism as wind moves seeds only short
distances, with maximum wind dispersal being sixteen feet (
|
|
WHAT PROBLEMS DOES IT CAUSE?
|
Dense infestations of yellow starthistle
displace native plants and animals, threatening natural ecosystems and nature
reserves. Yellow starthistle also significantly depletes soil moisture reserves
in annual grasslands in California (Gerlach unpubl. data) and in perennial
grasslands in Oregon (Borman et al. 1992). Long-term ingestion by horses causes
a neurological disorder known as chewing disease, a lethal lesion of the
nigropallidal region of the brain. This disease is expressed as a twitching of
the lips, tongue flicking, and involuntary chewing. Permanent brain damage is
possible, and affected horses may starve to death (Kingsbury 1964). Yellow
starthistle interferes with grazing and lowers yield and forage quality of
rangelands, thus increasing the cost of managing livestock (Roch̩ and Roch̩
1988). It can also reduce land value and limit access to recreational
areas.
åÊ
|
|
HOW DOES IT GROW AND REPRODUCE?
|
Plants reproduce only by seed and
generally flower from May to September. When adequate moisture is
available, yellow starthistle can survive as a short-lived perennial and
flower throughout fall, winter, and spring. However, the flowers produced
during winter are often killed by frost (Gerlach unpubl. data). Almost all
plants are self-incompatible and require pollen from a genetically
compatible plant to produce seed (Maddox et al. 1996). |
(click on photos to view larger image)
|
Centaurea solstitialis on left; C. melitensis on
right
European honeybees are an important pollinator, and in some
populations are responsible for 57 percent of seed set (Barthell unpubl. data).
Seeds produced per head (30-80) and flowerhead production per plant (1-1,000)
are variable, depending on soil moisture levels and intensity of competition
(DiTomaso, unpubl. data). Large plants can produce nearly 75,000 seeds. Seed
production in heavily infested areas varies between fifty to 200 million seeds
per acre. Studies of seed survival in soil have found significant survival to
ten years (Callihan et al. 1993). Seeds typically germinate in late fall or
early winter, when soil moisture is present (Maddox 1981) and overwinter as
basal rosettes.
Germination responses in yellow
starthistle are greatly reduced in dark environments and by exposure to light
enriched in the far-red portion of the spectrum (Joley 1995). The two types of
achenes also differ in response to light (Joley 1995). During early seedling
establishment, root growth is vigorous and can extend deeper than one meter (3.3
ft) (Roch̩ et al. 1994, DiTomaso unpubl. data), providing plants with access to
deep soil moisture reserves during dry summer months. Reduced light levels cause
the rosettes to produce fewer but larger leaves and to assume a more upright
growth form (Roch̩ et al. 1994). Reduced light levels also significantly reduce
root growth and flower production (Roch̩ et al. 1994). Consequently, survival
and reproduction are significantly reduced in shaded areas, and the plant is
probably less competitive in dense stands of established perennials. Bolting
occurs from late spring to early summer, and spiny flowerheads generally are
produced from early summer to late summer or fall. The spines on the flowerheads
may protect them from herbivory by large animals, but they do not prevent
significant herbivory by grasshoppers or seed predation by birds (Gerlach
unpubl. data).
åÊ
|
|
HOW CAN I GET RID OF IT?
|
It is important to prevent large-scale
infestations by controlling new invasions. Spot eradication is the least
expensive and most effective method of preventing establishment of yellow
starthistle. In established stands, any successful control strategy will require
dramatic reduction or, preferably, elimination of new seed production, multiple
years of management, and follow-up treatment or restoration to prevent rapid
reestablishment.
Effective control using any of the
available techniques depends on proper timing. Combinations of techniques may
prove more effective than any single technique. For example, prescribed burning
followed by spot application of post-emergence herbicides to surviving plants
can prevent the rapid reinfestation of the treated area. Similarly, combining
mowing and grazing, revegetation and mowing (Thomsen et al. 1996a, Thomsen et
al. 1996b), or herbicides and biological control may provide better control than
any of these strategies used alone. Effective combinations may depend on
location or on the objectives and restrictions imposed on land
managers.
åÊ
|
|
Physical control:
|
Mechanical methods: Tillage can control this
thistle; however, this will expose the soil for rapid reinfestation if
subsequent rainfall occurs. Under these conditions, repeated cultivation is
necessary (DiTomaso et al. 1998). During dry summer months, tillage practices
designed to detach roots from shoots prior to seed production are effective. For
this reason, the weed is rarely a problem in agricultural crops. Weedeaters or
mowing can also be used effectively. However, mowing too early, during the
bolting or spiny stage, will allow increased light penetration and more vigorous
plant growth and high seed production. Mowing is best when conducted at a stage
where 2 to 5 percent of the seed heads are flowering (Benefield et al. 1999).
Mowing after this period will not prevent seed production, as many flowerheads
will already have produced viable seed. In addition, mowing is successful only
when the lowest branches of plants are above the height of the mower blades.
Under this condition, recovery is minimized. Results should be repeatedly
monitored, as a second or perhaps a third mowing may be necessary to ensure
reduced recovery and seed production (Thomsen et al. 1996a, 1996b).
Prescribed burning: Under certain conditions, burning can
provide effective control and enhance the survival of native forbs and perennial
grasses (Robards, unpubl. data, DiTomaso et al. 1999a). This can be achieved
most effectively by burning after native species have dispersed their seeds but
before yellow starthistle produces viable seed (June-July). Dried vegetation of
senesced plants will serve as fuel for the burn. At Sugarloaf Ridge State Park
in Sonoma County, three consecutive burns reduced the seedbank by 99.5 percent
and provided 98 percent control of this weed, while increasing native plant
diversity and perennial grasses (DiTomaso et al. 1999a). No additional control
method was used in the fourth year. In that year, unfortunately, the seedbank of
yellow starthistle increased by thirty-fold compared to the previous year
(DiTomaso unpubl. data).
åÊ
|
|
Biological control:
|
Insects and fungi: Six USDA approved insect species that feed on
yellow starthistle have become established in California (Pitcairn 1997a and
1997b). These include three weevils, Bangasternus orientalis, Eustenopus
villosus, and Larinus curtus, and three flies, Urophora sirunaseva, Chaetorellia
australis, and C. succinea (Woods et al. 1995). All of these insects attack
yellow starthistle flowerheads, and the larvae utilize the developing seeds as a
food source. The most effective of these species are E. villosus and C. succinea
(Balciunas and Villegas 1999). With the possible exception of a few sites, the
insects do not appear to be significantly reducing starthistle populations, but
success may require considerably more time for insect numbers to increase to
sufficient levels.
Current evidence indicated a 50 to 75 percent reduction in seed
production in areas with significant bioagent populations (Pitcairn and Ditomaso
unpubl. data). A root-attacking flea beetle (Ceratapion brasicorne) is also
being studied (Pitcairn, pers. comm.). Researchers are seeking other
starthistle-specific foliar- and stem-feeding insects in Asia Minor. Research is
also currently being conducted on three native or naturalized fungal pathogens,
Ascochyta sp., Colletotrichum sp., and Sclerotinia sclerotiorum for the control
of yellow starthistle seedlings (Woods and Popescu 1997).
Grazing: Intensive grazing by sheep, goats, or cattle before the
spiny stage but after bolting can reduce biomass and seed production in yellow
starthistle (Thomsen et al. 1996a, 1996b). To be effective, large numbers of
animals must be used for short durations. Grazing is best between May and June,
but depends on location. This can be a good forage species.
Plant competition: Revegetation with annual legumes capable of
producing viable seed provides some level of control in pastures (Thomsen et al.
1996a, 1996b). In some areas subterranean clover (Trifolium subterraneum) proved
to be the best of sixty-six legumes tested. In other sites rose clover (T.
hirtum) and/or perennial grasses may be the preferred species. Control was
enhanced when revegetation was combined with repeated mowing (Whitson et al.
1987).
åÊ
|
|
Chemical control:
|
Although several non-selective pre-emergence
herbicides will control yellow starthistle, few of these can be used in
rangeland or natural ecosystems. The exception is chlorsulfuron, which provides
good control in winter when combined with a broadleaf selective post-emergence
compound. However, chlorsulfuron is not registered for use in rangelands or
pastures.
The primary options for control in non-crop areas are
post-emergence herbicides; 2,4-D, triclopyr, dicamba, and glyphosate (DiTomaso
et al. 1998). All but glyphosate are selective and preferably applied in late
winter or early spring to control seedlings without harming grasses. Once plants
have reached the bolting stage, the most effective control can be achieved with
glyphosate (1 percent solution). The best time to treat with glyphosate is after
annual grasses or forbs have senesced, but prior to yellow starthistle seed
production (May-June). The most effective compound for yellow starthistle
control is clopyralid (as Translineå¨), a broadleaf selective herbicide (DiTomaso
et al. 1998). Clopyralid provides excellent control, both pre-emergence and
post-emergence, at rates between 1.5-4 acid equivalent or 4-10 oz formulated
product per acre. Although excellent control was achieved with applications from
December through April, earlier applications led to significant increases in
quantity of other forage species, particularly grasses.
|